top of page
Girish Suryajoies
Principal UX Designer
Health Monitoring
role: Senior Design Consultant
team: 5
responsibility: Design Lead / Strategy / Designer Reviewer
duration: 12+ Months
Introduction
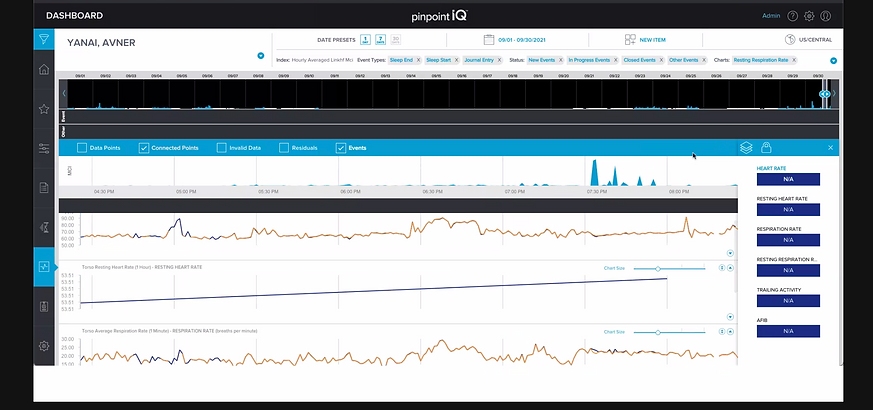
The PhysIQ clinical portal is a web, mobile, and tablet-based dashboard enabling clinicians to remotely monitor patient physiological data streamed from wearable biosensors. As a core part of the FDA-cleared pinpointIQ platform, it supports remote patient monitoring (RPM) and virtual care models such as post-acute care, hospital-at-home, and clinical trials.
Requirement

Goal
-
Gain foundational understanding of RPM and virtual care enablement
-
Capture the perspectives and workflow needs of clinician users
-
Assess the current PhysIQ clinician portal to uncover usability or workflow gaps
-
Identify and prioritize feature/functionality improvements focused on the event workflow (from event detection to intervention and documentation).
Double Diamond Process for Clinician Portal
Discover (Explore Problem)
-
Conduct interviews and shadow clinicians, nurses, and trial operators to observe real-world event workflows, pain points with current UI, and issues like alert fatigue.
-
Gather feedback on mobile/tablet usage, clinical trial needs, and documentation bottlenecks.
-
Benchmark innovative dashboards, timeline views, and notification strategies used in healthcare technology.
Define (Clarify Opportunity)
-
Synthesize insights from discovery into key problem statements:
- Too many unprioritized alerts overwhelm clinicians.
- Event data lacks contextual timeline and severity annotation.
- Dashboard layout is cluttered and role-agnostic.
- Mobile workflow isn't intuitive or fast for urgent actions.
- Trial teams need cohort views and regulatory template support. -
Prioritize design goals for the event timeline, severity prioritization, and real-time mobile access.
Develop (Generate Solutions)
-
Ideate and co-create annotated event timeline concepts, UI sketches for role-based dashboards, severity-based alert systems, and streamlined notification flows.
-
Prototype one-click intervention features (call, message, escalate), custom cohort documentation templates, and touch-optimized bedside tablet screens.
-
Test prototypes with end-users (clinicians, nurses, trial operators) for usability, clarity, and responsiveness.
Deliver (Implement & Refine)
-
Roll out validated features: event timeline view, severity-layered alerts, and mobile/tablet integration for bedside and remote care.
-
Launch user-centered dashboards tailored by clinical role, supported by flexible real-time data visualization and patient baseline comparisons.
-
Provide trial teams with study-based workflow grouping and regulatory-aligned templates.
-
Gather ongoing feedback, iterate quickly, and update features to improve event workflow, engagement, and compliance.

















Value additions
-
Use AI models to predict early deterioration risk (e.g., based on continuous biosensor data trends, AI flags a high probability of a future cardiac event before vitals cross thresholds).
-
AI can classify events automatically (low/medium/high severity) to prioritize clinician attention, reducing false alarms and alert fatigue.
-
Automatically generate concise clinical summaries of patient data trends and event logs, helping clinicians save time
in reviews. -
Move from static, population-level thresholds to individualized baselines where the AI dynamically adjusts thresholds based on patient-specific biometrics.
-
AI can suggest anomaly patterns in trial populations, improving early signal detection and reducing data review time for research teams.
-
Integrate a chatbot assistant for clinicians that can respond to queries
bottom of page